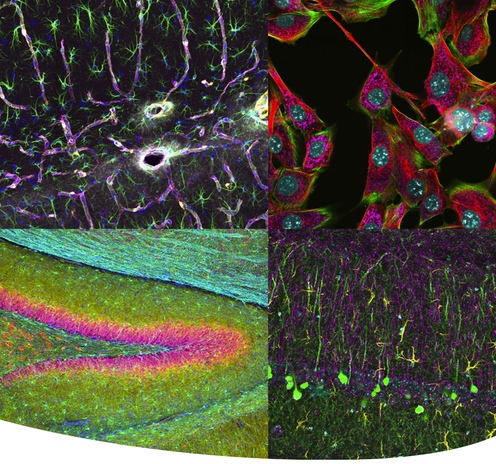
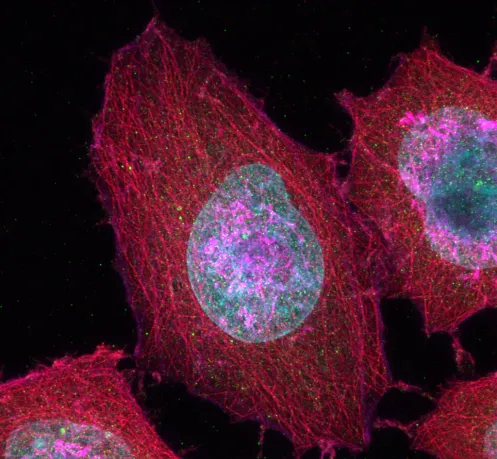
NEW Confocal microscope
OLYMPUS FV4000
Transforming Precision Imaging Our more than 100 years of optical expertise have led to the FV4000 microscope—a technological breakthrough that delivers profound image quality with the potential to change what you’re able to see and empower your research.
Olympus Confocal FV4000
- Superior image quality with our ultra-low-noise SilVIR™ detector and an industry-leading six channels, ten laser lines, and 400–900 nm dynamic range.
- Acquire confocal imagesup to 60x faster and super resolution images up to 8x faster than the FV3000.
- Count the number of photons in each pixeland represent features as discrete histograms of photons captured at different wavelengths.
- Game-changing dynamic range enables you to count from a few photons to thousands with linearity—a first in confocal microscopy.
- Easy to use with minimal adjustments required to obtain high-impact images and data.
AI Imaging
Stunning, Data-Rich Images in Less Time and with Less Effort
Get better images in less time and with less effort.
TruAI™ denoise technology takes the already low-noise images from the FV4000 and reduces noise to ultra-low limits for stunning, data-rich resonant images.
To speed up image analysis, you can pre-train an AI model so that the system can automatically segment your image data, greatly reducing the workload of this often time-consuming manual process.
Then,TruAI technology further streamlines the analysis so that you can get your data quickly.
Near-Infrared Imaging Solutions
Near-Infrared Imaging Solutions
See in red—open a spectrum of possibilities. When investigating cellular
processes in the context of their 3D environments, model organisms and
organoids are often superior to 2D culture systems. Near-infrared (NIR)
microscopy has proven beneficial for imaging biological tissues due to a
lower absorption and scattering of NIR light than visible light.
Easy-to-Acquire, Quantitative Confocal Data
The FV4000 confocal microscope uses our advanced, silicon-based SilVIR™ detector that makes it easier than ever to acquire precise, reproducible data.
SilVIR Next-Generation Detector Technology
The SilVIR detector combines two advanced technologies—a silicon photomultiplier (SiPM) and our patented* fast signal processing design.
- High dynamic range
- Low noise
- No degradation of sensitivity
- Less sensitivity variation among other detectors
SilVIR Detector Technology
Silicon Photomultiplier
The detector’s silicon photomultiplier consists of multipixel Geiger-mode-operated avalanche photodiodes (APD). It can detect random incident photons simultaneously, enabling a higher photon detection efficiency for a wider range of wavelengths and dynamic range. It also provides quantitative data—the height of the output pulse precisely shows the number of detected photons.
Patented* Fast Signal Processing
See Deeper into Your Samples with Our New Multi-Immersion Objective
Our new multi-immersion objective (LUPLAPO25XS) with groundbreaking new immersion technology lets you see deeper into your samples and reveal structures that were previously out of reach.
New Experience with Silicone Gel Pad
LUPLAPO25XS
NA0.85 / WD 2.00mm*
Immersion: Silicone gel pad, Silicone oil, Water
* approx. 1mm for silicone gel pad
Gentler High-Speed Time-Lapse Confocal Imaging
Time-lapse imaging is easier with smart features:
- Capture every moment of live cell dynamics: resonant scanner can acquire high-resolution images over a wide area
- Minimal phototoxicity: the scanner’s short pixel dwell time reduces the time the focused laser beam rests on a single spot
- Better signal-to-noise ratio: the SilVIR detector’s high sensitivity produces higher quality images at higher speeds
- Greater precision: rolling average processing maintains qualifications and time resolution
HeLa cells labeled by MitoView 720. XYZT imaging by 1K resonant scanner for 30 min.
Flexible Macro to Micro Imaging
The macro-to-micro workflow enables you to easily observe the target sample from the macro level—whole body or tissue—down to the cell or subcellular level.
Overview and edge images of a Drosophila wing (42-hour pupation). Stained with phalloidin (AlexaFluor 405, F-actin, Cyan), anti-phosphotyrosine antibody (AlexaFluor 555, cell surface, red), and anti-HRP antibody (AlexaFluor 647, axon, blue).
Sample courtesy of: Sun Zhengkuan, Shigeo Hayashi, Laboratory for Morphogenetic Signaling, RIKEN Center for Biosystems Dynamics Research, Japan.
High-Quality Optics for Efficient NIR Fluorescence Imaging
The FV4000 system's optical elements have a high transmission from 400 nm to 1300 nm, including the galvanometer and resonant scanner, which are coated in silver rather than aluminum.
Our award-winningX Line™ objectivesare corrected for chromatic aberrations between 400–1000 nm. They also have a higher numerical aperture, excellent flatness, and very high transmittance from UV to NIR, increasing the multiplexing capabilities.
For improved colocalization reliability, ourspecialized A Line™ (PLAPON60XOSC2) oil immersion objective(ne~1.40) significantly minimizes chromatic aberration for strict colocalization analysis.
High-Resolution 3D Images in Thick Samples
When imaging thicker samples, the FV4000 microscope
enables you to capture high-resolution, 3D images.
- Take advantage of NIR’s longer wavelength to penetrate deeper into tissue thanks to the SilVIR detector’s wide dynamic range and sensitivity
- Image deeper with less scattering and absorption by taking advantage of the fact that light-scattering compounds—like melanin and heme—absorb less light between 700–1500 nm
- Image significantly deeper than what’s possible with visible lasers thanks to 685 nm, 730 nm, and 785 diode lasers on the FV4000
- High NA silicone objectives minimize spherical aberration and silicone oil doesn’t dry out, both advantageous for time-lapse imaging
- Improve overall image quality and Z resolution using TruSight™ deconvolution for stunning 3D images of thick samples
- Enjoy a seamless workflow from acquisition to publication with specialized cellSens™ software algorithms
HeLa cell spheroid labeled by DAPI (cyan, cell nuclei) and AlexaFluor790 (magenta, Ki-67). Imaging of the spheroid’s whole volume was possible by NIR 785 nm, although only surface area cell nuclei observation was possible using a 405 nm laser.
Time-lapse image of HeLa cells stained with Hoechst33342 (nuclear, blue), MitoTracker Green (mitochondria, green), LysoTracker Red (Lysosome, yellow), SiR-Tubulin (tubulin, magenta), POR-SA-Halo (ER, cyan). Hoechst33342: Ex 405 nm/Em, MitoTracker Green: LysoTrakcer Red: SiR-Tubulin: POR-SA-Halo: Sample courtesy of: Masayasu Taki, Ph.D., Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Japan, Yuichi Asada and Ryusei Aruga, Graduate School of Science, Nagoya University, Japan.
Faster, Easier Image Analysis TruAI
Image analysis requires data extraction using segmentation techniques based on intensity value thresholds. However, this can be time-consuming and is affected by the sample conditions.
TruAI image segmentation using deep learning helps streamline image processing and minimize sample variables for more accurate image analysis. It enables you to segment superior performance with weak fluorescence fluorescence images or tissues that are usually difficult to extract using the simple thresholding method.
Configurations
The FV4000 microscope is engineered to be modular, making it easy for you to configure the system based on your applications and budget. You can start with a standard FV4000 and easily upgrade to multi photon imaging by adding the MPE module as your research changes.
One Platform for Your Research Needs
Multi photon and single-photon combination imaging in one sample is also possible. The FV4000MPE microscopes capable of second and third harmonic generation imaging, so different researchers or users can make the most out of the system. If your research requires a custom setup, the microscope’s modularity and optional ports enable you to customize the system to add extra lasers, cameras, detectors, and more.
FV4000MPE
Multiphoton Laser Scanning Microscope
Intuitive User-Interface and Workflows
The photomultiplier tubes traditionally used in confocal imaging require voltage adjustments depending on the sample’s brightness level as well as an offset adjustment to reduce signal noise. This requires expert knowledge and experience to make proper adjustments to acquire high-quality confocal images.
The SilVIR detector’s voltage is optimized for sensitivity and low noise at the factory, so you don’t need to make any voltage and offset adjustments—all you need to adjust is the laser power to achieve a certain photon number.
Since the signal-to-noise (S/N) ratio is proportional to the photon number, you can maintain consistent image quality by keeping the photon number constant.
Applications
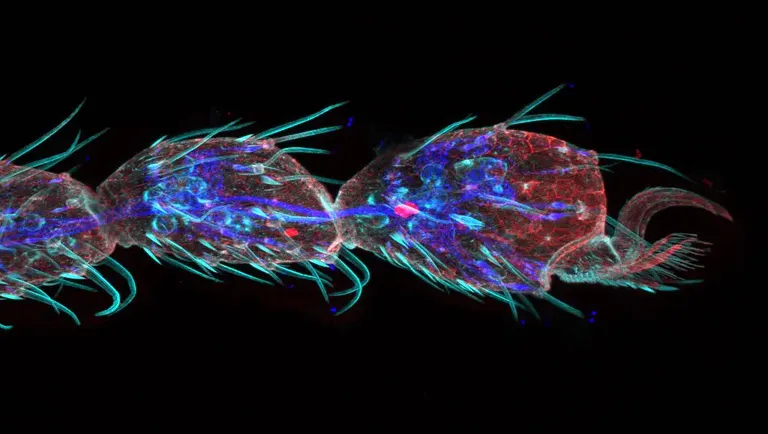
Gustatory hair and Peudotrachea in Drosophila (42hours pupation)
Stained with Phalloidin (AlexaFluor 405, F-actin, Cyan), Anti-phosphotyrosine antibody (AlexaFluor 555, cell surface, red) anti-HRP antibody (AlexaFluor 647, axon, blue).
Sample Courtesy of: Sun Zhengkuan and Shigeo Hayashi, Laboratory for Morphogenetic Signaling, RIKEN Center for Biosystems Dynamics Research, Japan.
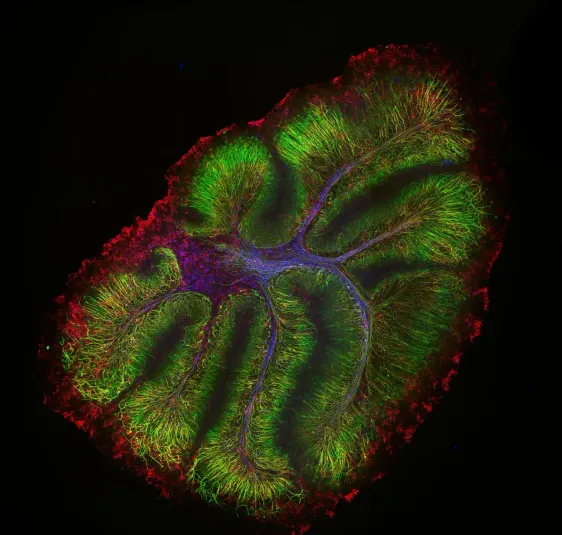
Neurofilament-heavy chain (NFH) in green, myelin basic protein (MBP) in red, glutathione S-transferase pi 1 (GSTpi) in blue. Mouse cerebellum captured with a UPLXAPO10X objective.
Sample courtesy of Katherine Given, Ph.D. Principal Investigator, Neurobiology University of Colorado Anschutz Medical Campus, Aurora, Colorado
Tip of a Drosophila leg (42-hour pupation), stained with phalloidin (AlexaFluor 405, F-actin, Cyan), anti-phosphotyrosine antibody (AlexaFluor 555, cell surface, red), and anti-HRP antibody (AlexaFluor 647, axon, blue).
Sample Courtesy of: Zhengkuan Sun, Shigeo Hayashi, Laboratory for Morphogenetic Signaling, RIKEN Center for Biosystems Dynamics Research, Japan.